Although Lyme borreliosis has been linked to hepatitis in early stages of infection, the association of chronic Borrelia burgdorferi infection with hepatic disease remains largely unexplored. We present the case of a 53-year-old woman diagnosed with Lyme disease who developed acute hepatitis with elevated liver enzymes while on antibiotic treatment. Histological examination of liver biopsy tissue revealed spirochetes dispersed throughout the hepatic parenchyma, and the spirochetes were identified as Borrelia burgdorferi by molecular testing with specific DNA probes. Motile spirochetes were also isolated from the patient’s blood culture, and the isolate was identified as Borrelia burgdorferi sensu stricto by two independent laboratories using molecular techniques. These findings indicate that the patient had active, systemic Borrelia burgdorferi infection and consequent Lyme hepatitis, despite antibiotic therapy.
Lyme disease (LD) caused by the spirochete Borrelia burgdorferi (Bb) and vectored by the deer ticks Ixodes scapularis and I. pacificus is the most common tick-borne illness in North America [1]. Early stages of LD are characterized by one or more of the following symptoms and signs: erythema migrans rash, headaches, stiff neck, arthralgias, myalgias, lymphadenopathy, and paresthesias [2] [3]. Neurological manifestations, cardiac involvement, and arthritis may be seen in later infection [2] [4] [5].
Elevated liver function tests (LFTs) are often reported in LD and can indicate hepatocellular injury and subclinical hepatitis [6]. Patients with early, disseminated Lyme disease are more likely to have abnormal LFTs than are patients with localized disease [7] [8]. Although there have been reports of granulomatous hepatitis associated with LD, this form of hepatitis has only been reported in early disease in the absence of antibiotic therapy [9] [10].
We present a case of granulomatous hepatitis associated with chronic LD. Although other causes of the hepatic inflammation were entertained, blood culture, histochemical staining and molecular testing revealed that the most likely cause of the liver disease was Bb infection.
A 53-year-old woman with chronic LD presented to her primary care physician with right upper quadrant pain and fatigue in January 2013. She had a history of a tick bite and subsequent erythema migrans (EM) rash in 1988, but she did not receive antibiotic therapy. In June 2000 she developed acute arthritis, and she was treated with corticosteroids and anti-inflammatory medications without benefit. Over the next decade she developed intermittent myalgias, hip pain, fevers, headaches, neck stiffness, memory loss, confusion and insomnia. In August 2010 a Lyme Western blot test was positive and the CD57 natural killer cell level was 18 cells/μl (normal, 60-360 cells/μl). Testing for tickborne coinfections was positive for Anaplasma phagocytophilum, and liver function tests were normal.
She was treated with oral antibiotics and her symptoms improved significantly over the next two years. The Lyme Western blot and A. phagocytophilum testing became negative in January 2012 and the patient discontinued antibiotic therapy, but in August 2012 her headaches, myalgias and arthralgias increased. She did not recall any recent tick bite. The Lyme Western blot was again positive and the CD57 NK level was 50 cells/μl. Liver function tests were normal. She was treated with clarithromycin and tinidazole, but in January 2013 she developed progressive right upper quadrant pain and fatigue. Her liver function tests were found to be significantly elevated (Table 1).
| Date | 4/24/12 | 8/14/12 | 1/15/13 | 1/23/13 | 1/28/13 | 2/15/13 | 3/25/13 | 5/29/13 | 7/16/13 | 9/26/13 | 1/24/14 | Normal Range* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdominal Pain | Onset | + | + | + | + | ± | ± | - | - | None | ||
| Musculoskeletal Pain | ± | ++ | + | ± | ± | ± | ± | ± | ± | ++ | ± | None |
| Clarithromycin | Start | Stop | Start | Ongoing | N/A | |||||||
| Tinidazole | Start | Stop | N/A | |||||||||
| Cefdinir | Start | Ongoing | N/A | |||||||||
| AlkPhos | 65 | 85 | 203 | 214 | - | - | 154 | - | 109 | 100 | 94 | 39-117 |
| AST | 22 | 25 | 156 | 232 | 199 | 179 | 96 | 57 | 56 | 34 | 19 | 0-40 |
| ALT | 25 | 26 | 315 | 439 | 384 | 316 | 159 | 84 | 68 | 47 | 14 | 0-32 |
| Bili | 0.5 | 0.5 | 0.5 | 0.8 | 1.0 | 0.8 | 0.8 | - | 0.8 | 0.4 | 0.5 | 0-1.2 |
| GGTP | - | - | 238 | - | 234 | 160 | - | 36 | - | - | - | 8-35 |
| Lyme WB | Pos | Pos | Pos | Neg | ||||||||
| A. phago IgM/IgG | Neg | Neg | Neg | |||||||||
| CD57 NK | 50 | 56 | 41 | 110 | 60-360 cells/ul |
Antibiotic therapy was discontinued, but the liver function tests remained elevated. Tests for hepatitis A virus, hepatitis B virus, hepatitis C virus, human immunodeficiency virus, herpes simplex virus, Epstein-Barr virus, syphilis, toxoplasmosis, cytomegalovirus, antinuclear antibodies, antimitochondrial antibodies and angiotensin converting enzyme were negative or normal. Abdominal ultrasound and MRI revealed normal liver architecture without focal lesions or biliary dilatation. A liver biopsy revealed granulomatous hepatitis with spirochetes noted in liver parenchyma (Figures).
The patient remained off antibiotics, but her symptoms of LD (primarily myalgias and arthralgias) worsened and her liver function tests remained elevated. The Lyme Western blot was positive and A. phagocytophilum testing was negative. She was started on clarithromycin and cefdinir in September 2013. As of January 2014 her LD symptoms had improved and her liver function tests had returned to normal. The CD57 NK level was 110 cells/μl and the Lyme Western blot remained positive.
Culture of Bb spirochetes was performed as described previously [11]. Ten mls of whole blood was collected by venipuncture and allowed to clot at room temperature for 10 to 15 minutes. Red blood cells (RBCs) were separated by centrifugation at 1,500 g for 15 minutes and the serum, buffy-coat and some RBCs were used for blood culture inoculum in Barbour–Stoner–Kelly H (BSK-H) complete medium, with 6% rabbit serum (Sigma Aldrich, #B8291) and the following antibiotics: phosphomycin (0.02 mg/l), rifampicin (0.05 mg/l), and amphotericin B (2.5 µg/l) (Sigma-Aldrich). Cultures were incubated at 32°C in an Oxoid anaerobic jar (Thermo Scientific) with an AnaeroGen sachet (Thermo Scientific), and they were checked weekly by light and/or dark-field microscopy for visible motile spirochetes. Cultures were concentrated by centrifugation at 15,000g for 20 minutes, the supernatant discarded, and the culture pellet was formalin-fixed for Dieterle and anti-Bb immunostaining or was covered with 200μl of Qiagen A1 lysis buffer (Qiagen).
The initial sectioning and examination of the liver biopsy was performed at Calgary Laboratories, Foothills Medical Center, Calgary, Alberta, using standard histological methodologies with the following stains: Hemotoxylin and Eosin, Periodic acid-Schiff, Periodic acid-Schiff diastase, Trichrome staining, Reticulin stain and Prussian Blue stain. Subsequently the stained mounted sections and unstained sections fixed on slides were forwarded to McClain Laboratories LLC, Smithtown, NY, for a second opinion and specialized staining for the detection of spirochetes.
Dieterle silver nitrate stain was performed at McClain Laboratories by standard histological methodology. Anti-Bb immunostaining and Bb molecular detection with 2 different Bb-specific DNA probes that fluoresce upon hybridization with complementary RNA or DNA (molecular beacons), Flagellin bb0147 (fla probe) and BBo 060 (probe 740) pre-engineered by Tyagi and colleagues [12], were conducted at McClain Laboratories. In addition, formalin-fixed culture pellets were paraffin-embedded and sectioned for Dieterle, anti-Bb immunostaining and staining with the DNA probes at McClain Laboratories, as described below.
Immunostaining of the liver biopsy sections and the culture pellet sections was performed using an unconjugated rabbit anti-Bb polyclonal antibody (Abcam ab20950) and then incubated with an alkaline phosphatase probe (Biocare Medical #UP536L) followed by a chromogen substrate (Biocare Medical #FR805CHC) and counterstained with Hematoxylin. Positive and negative controls for both Dieterle and anti-Bb immunostains were prepared for comparison purposes with liver sections from mice inoculated with Bb and uninfected mice, respectively. Staining was titrated to determine optimal antibody dilutions (1:400) to achieve positive staining of spirochetes while minimizing background staining. Negative controls of culture pellets from mixed Gram-positive bacteria and mixed Gram-negative bacteria were also prepared for comparison purposes to determine possible cross-reactivity with commonly encountered microorganisms.
Bb molecular beacon DNA probes were generously supplied by Dr. Alan MacDonald. DNA probe sequences were subjected to BLAST analysis as part of the initial design to determine specificity. Probe FlaB is derived from the Bb open reading frame (ORF) BB0147 of the flagellin B gene that contains more than 1000 nucleotides. A sequence of 23 nucleotides was selected from this ORF. A nucleotide Basic Local Alignment Search Tool (BLASTn) search of the 23 nucleotide sequence disclosed no matches in the human genome or in any other lifeform other than that of Bb BB0147. Probe 740 is derived from the Bb ORF BB740 that represents a Bb inner cell membrane protein. A similar BLASTn search produced no matches other than that of the Bb ORF BB740. Thus a high degree of specificity for Bb sensu stricto was assured.
Bb detection with the molecular beacons was performed by the following protocol: Paraffin sections were completely dewaxed by baking at 60°C and immersion in serial 100% xylene baths, followed by serial immersion through baths of 100% ethanol, 90% ethanol, 80% ethanol, and finally in distilled H2O, then air-dried. Fixed sections were immersed in 20 μl of the working DNA beacon. The sectioned specimen was covered with a thin layer of plastic cut from a Ziploc® freezer bag and was heated at 90°C for 10 minutes to denature all DNA and RNA. The heat was reduced to 80°C for 10 minutes, then samples were allowed to gradually cool to 24°C. The slides were washed in PBS, and covered with 30% glycerol and a glass coverslip, then examined under an EPI Fluor microscope. Staining with DNA probes was performed alongside staining of positive and negative controls. The positive control was prepared by embedding a known Bb strain in agarose, formalin-fixing the specimen then blocking in paraffin and staining sections as described above.
Blood culture pellets dissolved in 200μl of Qiagen buffer were forwarded to the University of New Haven, Department of Biology and Environmental Science, West Haven, CT, USA and Australian Biologics, Sydney, NSW, Australia for nested PCR detection of Bb targeted to the pyrG gene and for real-time PCR detection of Bb targeted to the 16S rRNA gene, respectively.
In order to genetically characterize the Bb strain, endpoint PCR amplification and sequencing of the Bb RPOC gene target from the blood culture isolate was performed by Australian Biologics followed by Basic Local Alignment Search Tool (BLAST) analysis for comparison with known Bb strain sequences.
Nested PCR was used to amplify the Borrelia pyrG gene from extracted DNA with the primers indicated in the table below. The first run of PCR used 20 µl of template DNA in a final reaction volume of 50 µl consisting of 1X Buffer B (Fisher Scientific) with 2 mM MgCl2, 0.4 mM dNTP mix, 2.5 U recombinant Taq polymerase (Invitrogen), and forward and reverse primers at a concentration of 2 µM each. The subsequent run used 1 µl of PCR product from the first reaction. All concentrations remained the same. Both reactions were cycled with the same protocol: 94ºC for 5 minutes, 40 cycles of 94 ºC for 1 minute, 48 ºC for 1 minute, and 72 ºC for 1 minute, with a final extension of 72 ºC for 5 minutes. Bb strain B31 DNA was used as a positive control, and water was used in place of DNA for a negative control.
| Outer primers | ATTGCAAGTTCTGAGAATA CAAACATTACGAGCAAATTC | 762 bp product |
| Inner primers | GATATGGAAAATATTTTATTTATTG AAACCAAGACAAATTCCAAG | 663 bp product |
Detection of Bb by PCR was performed using the Eco™ Real-Time PCR system with software version 3.0.16.0, as previously described [13], with primers AB-B1 targeting the Borrelia 16S rRNA gene. DNA was extracted from the blood culture pellet dissolved in 200 μl of Qiagen A1 lysis buffer using the QIAamp DNA Mini Kit (Qiagen). The extracted DNA sample was analyzed in duplicate with positive and negative controls, The thermal profile involved incubation for 2 mins at 50°C, polymerase activation for 10 mins at 95°C then PCR cycling for 40 cycles of 10 secs at 95°C dropping to 60°C sustained for 45 secs.
The magnitude of the PCR signal generated (∆R) for each sample was interpreted as positive or negative compared to positive and negative controls.
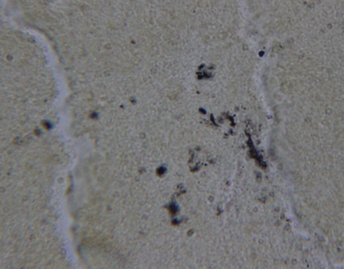
Dieterle staining of the blood culture pellet demonstrated both long slender spirochetes ranging from approximately 0.1 to 0.5 μm in width and approximately 2 to 6 μm in length, some with visible helices, and round morphological variants ranging from approximately 0.5 to 2 μm in diameter that looked much like some of the morphological variants seen in the liver section (Figure 1).
Anti-Bb immunostaining of the blood culture was strongly positive, exhibiting a bright red cherry color (Figure 2). There was some positive staining of cellular debris, possibly because of the antigens released from lysed spirochetes or secreted by Bb. Strongly positive staining was not observed in the culture pellets of control microorganisms.
Molecular beacon staining of the culture pellet sections was strongly positive throughout (Figure 3). Culture pellets contained both cultured bacteria and human cellular debris (intact red blood cells and lysed blood cells) indicating that Bb nucleic acid sequences were present throughout the cellular debris and within intact RBCs.
The initial histological examination was performed at Calgary Laboratories and yielded a tentative diagnosis of primary biliary cirrhosis. Normal hepatic architecture was reported with some portal triads expanded by dense mixed inflammatory filtrate, mostly centered close to the bile ducts. The infiltrate contained lymphocytes, plasma cells, occasional eosinophils and prominent non-necrotizing granulomata. Occasional intraepithelial lymphocytes were noted within bile ducts and were associated with reactive changes of bile duct epithelium. Most periportal infiltrates were circumscribed and limited to portal triads with minimal interface activity. Some lymphocytes, plasma cells, and epithelioid granulomata were present in the lobules, although no active hepatocyte necrosis was noted. No significant fibrosis, stainable iron, or diastase-resistant intracellular globules were reported.
Further histological studies at McClain Laboratories included both examination of the stained slides forwarded by Calgary Laboratories and examination of slides stained to detect spirochetes, specifically Bb (Dieterle, anti-Bb immunostaining, and the molecular beacons, probe 740 and probe FlaB). Portal inflammation with an infiltrate of lymphocytes, plasma cells and histiocyte granulomas was observed as previously reported. No macrophages and neutrophils were evident, which suggested a chronic process. As reported previously by Calgary Laboratories, there was no evidence of hepatocyte or biliary duct epithelial cell necrosis; however, collapse of reticulin and fibrosis was observed on Trichrome stain and intracellular mulberry-shaped granules were observed within Kupffer cells on PASD stain. The collapse of reticulin and fibrosis also pointed towards chronicity, but fell short of cirrhosis.
Dieterle silver nitrate staining of the liver biopsy tissue provided additional information that was not apparent on the original slides (Figure 4). Dieterle staining revealed well-defined spirochetes scattered throughout much of the section, predominantly in the parenchyma, and tiny vacuolated or granular forms within Kupffer cells. Dieterle staining also yielded positive dots within the mulberry-shaped granules inside Kupffer cells that had been observed on PASD stain. Less positive staining was observed around the bile ducts where plasmacytic-lymphocytic inflammation was present along with occasional eosinophils.

The negative control showed no significant immunostaining (Figure 5A), while the positive control using a known Bb strain resembled the biopsy slide (Figure 5B). Staining of the biopsy slide resulted in intense red staining of microorganisms, some of which demonstrated typical spirochetal morphology (Figure 5C and 5D). The section demonstrated strongly positive patches of staining visible in the parenchyma between hepatocytes. Immunostaining predominantly stained vacuolated Kupffer cells, failing to reveal well defined spirochetes; however, within or closely related to patches of positive intercellular staining, round, oval, and some curvy, elongated, positively-stained shapes were noted.

The negative control showed no significant molecular hybridization (Figure 6A), while the positive control using a known Bb strain resembled the biopsy slide (Figure 6B). Staining with the DNA molecular probes, probe 740 and probe Fla B, demonstrated granular Bb profiles similar in appearance to those seen in the Dieterle stain (Figure 6C and 6D). Both cytoplasmic and nuclear fluorescent signals were observed within hepatocytes, representing probe hybridization with Bb nucleic acids derived from cytoplasmic and nuclear granular infection.
Nested PCR analysis targeted to the pyrG gene performed at University of New Haven and real-time PCR analysis targeted to the 16S rRNA gene performed at Australian Biologics were positive for Borrelial DNA in the blood culture pellets.
BLAST analysis performed at Australian Biologics characterized the PCR isolate as Bb sensu stricto with the closest matches at 96% to Bb sensu stricto strains CA382, N40, ZS7, and B31, and the next closest match at 95% to Bb sensu stricto strain JD1-1 (Figure 7). The isolate was significantly divergent from B. garinii sequences with homologies of 90-91%.
Our patient had evidence of granulomatous hepatitis associated with culture of motile spirochetes in blood samples and detection of various forms of Bb in liver biopsy sections. The hepatitis occurred in the setting of chronic Lyme disease and treatment with antibiotics. Although coinfection with A. phagocytophilum was present earlier in the course of disease, testing for this organism was repeatedly negative at the time that hepatitis supervened. The onset of hepatitis corresponded with initiation of tinidazole treatment, but the abnormal LFTs persisted for months after the antibiotic was discontinued. Thus the antibiotic may have played a role in triggering the hepatitis, but persistent Bb infection appeared to be the primary cause of liver damage in our patient.
In Lyme disease a sequence of events takes place in the liver. Hepatic Kupffer cells rapidly take up Bb spirochetes after the organisms enter the circulatory system [14], and liver injury results from hepatic invasion by the Bb spirochetes provoking a cellular and humoral immune response and resulting in abnormal LFTs [15] [16]. Histological evidence of Bb infection in the liver includes sinusoidal infiltration by a mixed inflammatory infiltrate, Kupffer cell hyperplasia, microvesicular fat deposits, hepatocyte ballooning and Bb spirochetes distributed within hepatic sinusoids and throughout the parenchyma [16] [17].
In our case study, Dieterle and anti-Bb immunostaining revealed spirochetes and granular morphological variants that indicated infection was present primarily in the hepatic parenchyma, rather than in and around the bile ducts where the inflammatory process was more obvious. Dieterle staining revealed distinct spirochetes throughout the parenchyma and tiny vacuolated or granular forms within Kupffer cells. Dieterle staining also yielded positive dots within the mulberry-shaped granules inside Kupffer cells that had been observed with PASD staining. Staining with the two DNA probes revealed both cytoplasmic and nuclear fluorescent signals within hepatocytes, indicating cytoplasmic and nuclear granular infection. The inflammatory process visualized in our case study clearly implied a chronic condition.
Staining with the DNA probes provided additional confirmatory evidence that the spirochetes seen in the liver sections were Bb, as the molecular beacons will not hybridize with nucleic acid sequences unless there is a 100% match for complementary DNA or derived RNA sequences [12]. Thus the fluorescent signal seen in the stained liver section was due to a 100% sequence homology in the detected target material.
Morphological variation of Bb was observed in the liver biopsy sections and included granular, round, spiral and helical forms. Variable forms of Bb are well documented in the medical literature [18] [19]. Reproductive propagules described as coccoid bodies, globular bodies, spherical bodies, granules, cysts, L-forms, spheroplasts or vesicles are induced by unfavorable growth conditions [20], and morphological variation may aid in immune evasion [19] [21]. We hypothesize that the morphological variants observed in the liver biopsy of this patient may have been partially responsible for antibiotic resistance and refractory illness.
Although there may have been an autoimmune component involved in the pathology of our case study, there was a substantial Bb spirochetal load throughout the hepatic parenchyma. An inflammatory response directed to Bb could therefore account for much of the liver damage seen in this patient. Although an initial diagnosis of primary biliary cirrhosis (PBC) was entertained, the patient did not demonstrate serological findings typical of PBC with negative testing for antimitochondrial antibodies and antinuclear antibodies. Therefore, antigenic mimicry between borrelial antigens and mitochondrial antigens does not alone appear to be the mechanism for inflammatory damage.
Despite evidence to the contrary, some researchers maintain that there is no “credible scientific evidence” for chronic Lyme disease due to persistent Bb infection following antibiotic therapy [22] [23] [24]. In contrast, we have demonstrated persistent Bb hepatic infection despite aggressive antibiotic treatment. Motile spirochetes were detected in the patient’s blood culture, and the blood culture isolate was identified as Bb sensu stricto by PCR testing. The positive blood culture demonstrated that the Bb spirochetes were viable and circulating, supporting the concept of chronic Lyme disease due to persistence of these organisms.
In summary, we describe a case of granulomatous hepatitis associated with chronic Lyme disease. The findings of motile spirochetes in blood culture and various morphological forms of Bb within the liver despite antibiotic therapy provide evidence that Lyme disease may be a chronic infection that affects different organs at different times and in different ways. Improved antibiotic treatment for chronic Lyme disease should become a priority for future investigation.
Funding Source: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors thank Drs. Stewart Adams, Gordon Atkins, Robert Bransfield, George Chaconas, Douglas Demetrick, Dorte Dopfer, Christopher Hardy, Nick Harris, Doug Kahn, Jyotsna Shah, Leo Shea, Janet Sperling and Carolin Teman for helpful discussion. We are grateful to Dr. Robert B. Allan and Guinnevere Stevens for technical support, and we thank Lorraine Johnson for manuscript review.
- Burgdorfer W, Barbour A, Hayes S, Benach J, Grunwaldt E, Davis J. Lyme disease-a tick-borne spirochetosis?. Science. 1982;216:1317-9 pubmed
- Steere A, Malawista S, Hardin J, Ruddy S, Askenase W, Andiman W. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med. 1977;86:685-98 pubmed
- Steere A, Bartenhagen N, Craft J, Hutchinson G, Newman J, Rahn D, et al. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99:76-82 pubmed
- Reik L, Steere A, Bartenhagen N, Shope R, Malawista S. Neurologic abnormalities of Lyme disease. Medicine (Baltimore). 1979;58:281-94 pubmed
- Steere A, Batsford W, Weinberg M, Alexander J, Berger H, Wolfson S, et al. Lyme carditis: cardiac abnormalities of Lyme disease. Ann Intern Med. 1980;93:8-16 pubmed
- Kazakoff M, Sinusas K, Macchia C. Liver function test abnormalities in early Lyme disease. Arch Fam Med. 1993;2:409-13 pubmed
- Horowitz H, Dworkin B, Forseter G, Nadelman R, Connolly C, Luciano B, et al. Liver function in early Lyme disease. Hepatology. 1996;23:1412-7 pubmed
- Benedix F, Weide B, Broekaert S, Metzler G, Frick J, Burgdorf W, et al. Early disseminated borreliosis with multiple erythema migrans and elevated liver enzymes: case report and literature review. Acta Derm Venereol. 2007;87:418-21 pubmed
- Chavanet P, Pillon D, Lancon J, Waldner-Combernoux A, Maringe E, Portier H. Granulomatous hepatitis associated with Lyme disease. Lancet. 1987;2:623-4 pubmed
- Zanchi A, Gingold A, Theise N, Min A. Necrotizing granulomatous hepatitis as an unusual manifestation of Lyme disease. Dig Dis Sci. 2007;52:2629-32 pubmed
- Bankhead T, Chaconas G. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol Microbiol. 2007;65:1547-58 pubmed
- Tyagi S, Kramer F. Molecular beacons in diagnostics. F1000 Med Rep. 2012;4:10 pubmed
- Mayne P. Investigation of Borrelia burgdorferi genotypes in Australia obtained from erythema migrans tissue. Clin Cosmet Investig Dermatol. 2012;5:69-78 pubmed
- Schaible U, Gay S, Museteanu C, Kramer M, Zimmer G, Eichmann K, et al. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am J Pathol. 1990;137:811-20 pubmed
- Goellner M, Agger W, Burgess J, Duray P. Hepatitis due to recurrent Lyme disease. Ann Intern Med. 1988;108:707-8 pubmed
- Duray P, Steere A. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65-79 pubmed
- Kurtti T, Munderloh U, Johnson R, Ahlstrand G. Colony formation and morphology in Borrelia burgdorferi. J Clin Microbiol. 1987;25:2054-8 pubmed
- Mursic V, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W. Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants. Infection. 1996;24:218-26 pubmed
- Stricker R, Johnson L. Lyme disease: the next decade. Infect Drug Resist. 2011;4:1-9 pubmed
- Stricker R, Johnson L. Persistent infection in chronic Lyme disease: does form matter? Res J Infect Dis. 2013;1:2.
- Stricker R, Johnson L. Lyme disease: call for a "manhattan project" to combat the epidemic. PLoS Pathog. 2014;10:e1003796 pubmed